Depending on the model of your inspire system you may be able to undergo mri on the head and extremities provided certain conditions and precautions are followed.
Inspire sleep apnea device mri safety.
Like cpap an inspire doctor can print out a usage record of inspire to show the medical examiner that you are using your prescribed sleep apnea treatment.
If so they are eligible for mri head and extremity scans provided specific guidelines in the manual are followed.
However they are eligible to have ct scans x rays and ultrasound images taken anywhere in the body.
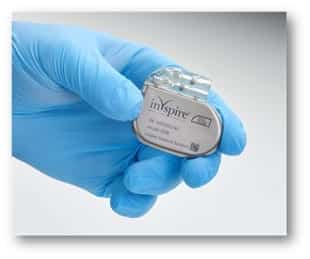
It is the only fda approved obstructive sleep apnea treatment that works inside the body with just the click of a button.
Important safety information inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients with an apnea hypopnea index ahi between 15 and 65.
Inspire upper airway stimulation uas is used to treat a subset of patients with moderate to severe obstructive sleep apnea osa apnea hypopnea index ahi of greater than or equal to 15 and less than or equal to 65.
Untreated obstructive sleep apnea osa has a negative impact on sleep and the body s ability to recover during sleep.
A wire is placed in the chest wall.
This device might help to stimulate the hypoglossal nerve that causes the throat and relevant muscles to move and open the airway for the user to breathe better.
Minneapolis june 5 2017 inspire medical systems inc manufacturer of the only fda approved implantable device for obstructive sleep apnea osa has received u s.
Inspire sleep apnea therapy review final verdict this device is suitable for people who are above the age of 18 years and suffer from moderate to severe obstructive sleep apnea.
By proving compliance there should be no issues renewing your commercial driver s license.
As a result osa patients may experience a high degree of daytime sleepiness.
It should be used after you have tried positive airway pressure treatments and they have not worked or you could not tolerate them.
Please check your inspire patient id card to determine if you are able to get an mri under specific conditions.
Food and drug administration fda approval for its next generation device the inspire 3028 implantable pulse generator which includes magnetic resonance mr conditional labeling allowing patients to undergo magnetic resonance imaging mri safely.
An implanted device called inspire.
There is a yellow mr triangle on the back of the patient id card if your patient has the inspire generator model 3028.
Inspire uas is used in adult patients 22 years of age and older who have been confirmed to fail or cannot tolerate positive airway pressure pap treatments such as continuous positive.
Inspire therapy has not been tested in people with a bmi greater than 32.